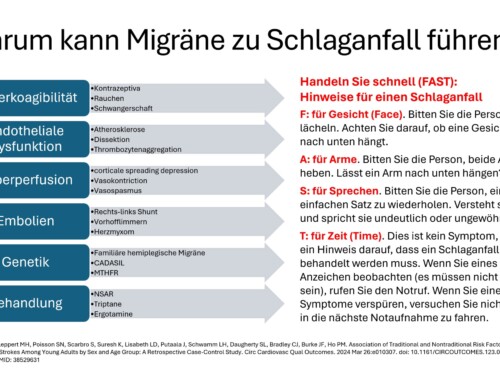
According to current knowledge, migraine pain is based on a neurogenic inflammatory reaction in the arteries of the meninges.
Inflammatory substances are released there in the initial stage of the migraine attack. These lead to increased pain sensitivity in the meninges with swelling and expansion of the vessel walls. Every pulse beat leads to a throbbing, pounding migraine pain, every movement of the head hurts due to allodynia and hyperpathy and increases the symptoms. During an attack, migraine patients therefore try to remain as calm and shield themselves from stimuli as possible and to avoid physical activity and vibrations. In recent years it has been possible to develop specific antibodies against messenger substances that cause inflammation during migraine attacks. The focus is on the Calcitonin Gene-Related Peptide, or CGRP for short. It is a neuropeptide consisting of 37 amino acids and encoded by the identical gene as the hormone calcitonin. CGRP is one of the strongest vasodilators and plays an important role in the development of migraines. If you give so-called monoclonal antibodies, the effects of these inflammatory substances can be stopped for a few weeks and the likelihood of migraine attacks can be reduced. Four antibodies have been developed and tested in numerous studies: erenumab (AMG 334), galcanezumab (LY2951742), fremanezumab (TEV-48125) and eptinezumab (ALD403).
- Erenumab (Aimovig®) was the first representative of this new class of active ingredients to be approved in Germany in July 2018 and has been available in pharmacies since November 2018. A legal dispute is putting a strain on Novartis' cooperation with the US biotech company Amgen, which developed erenumab. On April 4, 2019, the companies accused each other in court of violating a cooperation agreement with Aimovig. Amgen has terminated the migraine collaboration agreement for erenumab for this reason. Amgen accuses Novartis of violating this agreement because Novartis is allegedly working with a competitor on a drug similar to Aimovig. Novartis denies this.
- The second antibody Galcanezumab (Emgality®) for migraine prophylaxis became available in pharmacies in Germany on April 1, 2019. Galcanezumab has been approved in the EU since November 2018.
- The third antibody, fremanezumab (Ajovy®), was also recommended for approval by the EMA's Committee for Medicinal Products for Human Use and approved by the European Commission in April 2019. The date of availability in pharmacies is still open. Ajovy differs from Aimovig and Emgality, among other things, in the application interval: In addition to the monthly application, a quarterly application is available.
The antibodies now available have all proven their effectiveness in very large international studies. There are antibodies that work against the ligand CGRP (galcanezumab, fremanezumab, eptinezumab) or block the receptor for CGRP (erenumab). CGRP is considered to play an important role in the development of migraines. Increased CGRP levels are found in migraine attacks, and in chronic migraines also between attacks. Intravenous administration of CGRP can cause migraine-like headaches in migraine patients. The triptans, which are effective in acute migraine therapy, inhibit the release of CGRP. Finally, the so-called Gepanten was able to show that by inhibiting the CGRP receptor, migraine attacks can be interrupted acutely and, if taken regularly, they can be prevented.
Fremanezumab was studied in a total of 2,000 patients in pivotal studies. The phase III Halo EM study examined patients with episodic migraine and the phase III Halo CM study examined patients with chronic migraine. The Halo CM study compared the effectiveness of 225 mg fremanezumab in monthly doses and 675 mg fremanezumab in quarterly doses compared to placebo in 1,130 patients. On average, patients suffered from migraines on 13.2 days per month (monthly fremanezumab group), 12.8 days per month (quarterly fremanezumab administration), and 13.3 migraine days per month (placebo group). In the group of patients receiving quarterly fremanezumab, 675 mg of fremanezumab was initially administered subcutaneously. After a period of 4 and 8 weeks, placebo doses were used. Patients receiving monthly fremanezumab were treated with a starting dose of 675 mg fremanezumab. They then received 225 mg of fremanezumab in week 4 and week 8. The main outcome measure was the reduction in average headache days per month. A headache day was defined by at least 4 hours of discomfort per day or the use of migraine-specific acute medication per day.
- The most significant reduction was observed in the monthly dosing group. In this group, patients had an average of 4.6 fewer days of migraines per month. 41% of patients achieved a halving of headache days per month with this application method.
- When fremanezumab was administered at 3-month intervals, an improvement in monthly migraine days of 4.3 days was observed. In this group, 38% of patients achieved a 50% reduction in headache days.
- In comparison, a reduction in headache days per month of 2.5 days was found in the placebo group. 18% in the placebo group showed a halving of headache days per month.
The approval text envisages the use of fremanezumab (as well as erenumab and galcanezumab) in adult patients who suffer from migraines on at least 4 days per month. The antibodies are approved for the preventive treatment of both episodic and chronic migraines. Fremanezumab is the only antibody available to date that has demonstrated significant effectiveness even when administered quarterly at 675 mg in three times the monthly dose of 225 mg. There is currently no special three-month syringe available that allows patients to receive the corresponding dose of 675 mg in just one application. Therefore, patients currently have to use 3 injections, even if they are used for three months, albeit at a single time.
The most common undesirable side effect was pain at the injection site. These did not differ significantly between the group treated with fremanezumab or placebo.
When using monoclonal antibodies to prevent migraines, some special considerations must be taken into account:
- Monoclonal antibodies are very expensive drugs. There are currently no comparative studies on previous guideline-based preventative migraine medications. If you compare the effectiveness data with the previous active ingredients, the average values do not show any particular superiority in terms of effectiveness. The special additional benefit lies in the fact that patients who did not respond to the previously available drugs, who had contraindications or who could not tolerate them, can still achieve effectiveness with the monoclonal antibodies. For reasons of cost-effectiveness, the new treatment options will therefore have to be used for patients for whom the previous guideline-based migraine prophylactics were not helpful.
- The monoclonal antibodies must be administered as an injection under the skin using an autoinjector.
- The monoclonal antibodies have a very long duration of action with a half-life of around four weeks. This means that after a month, around 50% of the active ingredient is still circulating in the bloodstream and is active. Since this is a very new class of medication, long-term effects from prolonged use cannot yet be assessed with certainty.
- Immunotherapy for the preventive treatment of migraines is not a “final cure” for migraines. Only about 30% of patients who have not responded adequately to previous preventative treatment measures can expect a reduction in migraine attacks by 50% or more. This effect is unlikely to occur in 70% of treated patients. A majority of patients who achieve this effectiveness will still experience migraine attacks that require acute medication treatment.
- Migraine is a complex disease. It requires an adaptation of one's lifestyle to the particular genetically determined willingness to react with migraine attacks. Knowledge, information, adjustment of behavior through regulation of the daily routine, breaks, relaxation, exercise, nutrition and stress reduction are essential prerequisites for controlling the course of migraines.
Migraines are the third most common human illness after tooth decay and tension-type headaches. It is the most severely disabling disease up to the age of 50. It particularly affects productive life at school, during studies, in training, at work, when starting a family and in social activities. For the scientific community, migraine has traditionally been a stepchild. There has been little progress in the understanding and treatment of migraine throughout human history. This is now changing significantly for the first time in our generation. Further scientific studies with specific insights into the development and treatment of migraine, as well as public awareness and research efforts, are needed. The basis for improved care are specialized care centers that can treat severely affected patients in a multimodal, multidisciplinary and contemporary manner.







I am a chronic cluster headache patient.
For the last 2.5 years I have had multiple pain attacks every day around the clock.
To be more precise, I wanted to die!
I have been receiving Ajovi antibody injections for 4 months.
I haven't had a headache for 3 months.
Previously, I took nasal triptans up to 3 times a day and therefore had a very high risk of a stroke.
Ajovi gave me my life back :-)
I took Aimovig for six months. Due to digestive problems we have now switched to Ajovy. Both remedies worked really well for me from the first time I took them! I'm down to about 0 to 1 migraine day per month and my tension headaches are almost gone. If I ever have a slight headache, “just” an aspirin helps. That was never the case before. In fact, the days after taking Ajovy I have enormous energy. Since then I have been able to exercise regularly again.
Thank you for these remedies – I have a completely normal, relaxed life again!!
I've been taking Ajovy for 2 months and haven't had a migraine attack since then, previously about 8-10/month. Only tiredness on the first two days after the injection. Finally without pain, I hope it stays that way, the quality of life is back!
I've been taking the medication for three months and I'm a new person. I have also suffered from chronic migraines since my early childhood. There is no therapy that I haven't tried. Ajovy works great for me. I was also able to reduce my pain days from 15 to 3, starting from the first injection. I am so grateful and happy and hope that it helps many other patients!
Great article and also agrees with my personal experience with fremanezumab!
This article is the first I've read on the subject that doesn't treat the drug as a miracle cure.
It's not one that I don't mean to say that it's not an effective drug. I am 48 years old, have had migraines since I was 5 years old and have had 30 pain days/month with an average of 22 migraine attacks/month for about fifteen years. I've had everything from beta blockers to acupuncture to Botox etc. without success. In the end I was left with a disability pension. I've been taking Ajovy for three months and unfortunately it hasn't had any effect. I hope that the euphoria for this medication is confirmed and that it helps as many patients as possible. However, I am critical of the fact that the long-term consequences of the medication are not at all predictable.