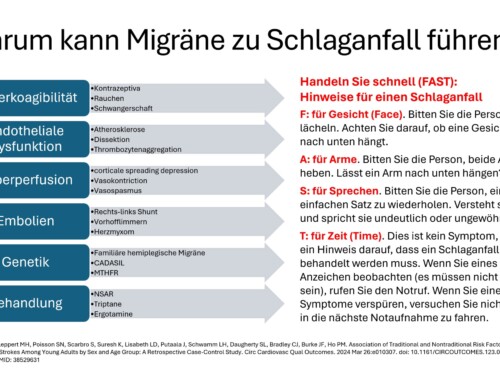
On November 11, 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) in Amsterdam gave a positive opinion recommending the granting of a marketing authorization for the medicine Vyepti for migraine prophylaxis. The applicant for this medicinal product is H. Lundbeck A/S. Vyepti will be available as a 100 mg concentrate for solution for infusion. The active ingredient in Vyepti is eptinezumab, a humanized monoclonal antibody, a migraine prophylactic (ATC code: N02CD05) that works by preventing the activation of CGRP receptors.
The benefit of Vyepti is a reduction in monthly migraine days. The most common side effects are nasopharyngitis and hypersensitivity. The full indication is: Vyepti is indicated for the prophylaxis of migraine in adults who experience at least 4 migraine days per month.
Vyepti should be prescribed by doctors who have experience treating migraines.
Detailed recommendations for the use of this medicine are described in the Summary of Product Characteristics (SmPC), which is published in the European Public Assessment Report (EPAR) and is available in all official languages of the European Union after the European Commission has granted marketing authorization .
Eptinezumab binds to the calcitonin gene-related peptide (CGRP). The drug prevents CGRP from binding to its receptor. This prevents migraine attacks. Vyepti is the first migraine-preventing antibody given by intravenous administration. The advantage is that rapid therapeutic drug levels are achieved in the blood. The effectiveness can occur as early as the day of the infusion. The infusion takes place within 30 minutes. It is administered every 12 weeks. This enables patients with migraines to receive preventive therapy with 4 treatments per year.
The efficacy, tolerability and safety of Vyepti were demonstrated in two Phase III clinical trials, PROMISE-1 in episodic migraine1 and PROMISE-2 in chronic migraine2. Significant effects were achieved for the primary endpoints of decrease in mean monthly migraine days (MMD) over weeks 1 to 12 in both episodic and chronic migraine. The mean reduction in MMD was around 4 migraine days per month for episodic migraine and around 8 days per month for chronic migraine. Effectiveness versus placebo was observed for both doses of Vyepti as early as the first day post-infusion. The safety of Vyepti was evaluated in 2,076 adult patients. Most common adverse reactions (≥2% and at least 2% or more than placebo) were nasopharyngeal inflammation and hypersensitivity. 1.9% of patients treated with Vyepti discontinued treatment due to side effects.
Source: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/vyepti







That's great news.
How long does it usually/probably take before you can actually get the medication? Is it more like a few months or years? Thanks!