 Mild migraine attacks during pregnancy should, if possible, be treated non-medication by means of isolation, rest, relaxation and ice packs.
Mild migraine attacks during pregnancy should, if possible, be treated non-medication by means of isolation, rest, relaxation and ice packs.
Even outside the maternity protection periods before and after birth, the Maternity Protection Act obliges companies to protect pregnant women and breastfeeding mothers and to carry out an individual risk assessment. If adequate protective measures are not possible, they must issue the employees operational bans on employment.
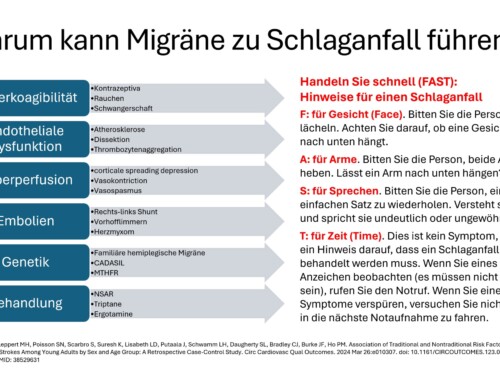
If there is a high frequency of attacks and severe attacks, an individual ban on employment can be issued by a doctor in accordance with Section 16 of the Maternity Protection Act (MuSchG). All licensed doctors, from gynecologists to neurologists, can issue the required medical certificate. There can be a variety of reasons for an individual employment ban. These include, for example, severe constant pain, high frequency of attacks and severe nausea. Physical and psychological stress caused by loud noise, significant pressure or stress can also play a role in the assessment. The treating doctor decides whether a ban on employment is medically necessary based on the medical circumstances.
Women who experience severe nausea and vomiting during pregnancy have a poor quality of life and an increased risk of maternal and fetal complications. For nausea and vomiting, metoclopramide can be used throughout pregnancy. In cases of severe nausea and ineffectiveness of metoclopramide, ondansetron can be used under strict indications during the second and third trimester of pregnancy. (1).
If there is urgent medical need, migraine attacks in the 1st and 2nd trimester of pregnancy can be treated with acetylsalicylic acid, ibuprofen or metamizole (2). These substances should be avoided in the 3rd trimester of pregnancy. A large study examined associations between in utero exposure to five over-the-counter analgesics (acetaminophen, ibuprofen, aspirin, diclofenac, naproxen) and adverse neonatal outcomes. Taking over-the-counter painkillers during pregnancy was associated with a significantly higher risk of adverse perinatal health outcomes in the children. Taking paracetamol in combination with other non-steroidal anti-inflammatory drugs was associated with the highest risk (3). Emerging evidence suggests that intrauterine acetaminophen exposure may be associated with genitourinary/reproductive disorders in offspring (4, 5). Studies suggest a possible association between prenatal acetaminophen exposure and an increased risk of neurodevelopmental disorders, atopy, and reproductive disorders (6). Paracetamol is not approved for severe pain (off-label); it is not expected to be effective for severe migraine attacks, even during pregnancy. It should only be considered during pregnancy if there is an urgent need for treatment and no other options are available. Pregnant women should be warned about the risks early in pregnancy. You should avoid paracetamol unless its use is urgently medically indicated. Treatment should be given at the lowest dose for as short a period as possible and not in combination with other medicines. Pregnant women should consult a doctor and not self-medicate with paracetamol (5).
In exceptional cases, single doses of metamizole may be acceptable during the first and second trimesters of pregnancy for severe pain when no other treatment options exist (7). Taking it during the third trimester of pregnancy can be associated with foetotoxic effects (restriction of kidney function, constriction of the ductus arteriosus), which is why the use of metamizole in the third trimester of pregnancy is contraindicated (8-10).
To date, there is no epidemiological evidence that triptans cause malformations or other complications during pregnancy (11, 12). The results of several pregnancy registries are available for sumatriptan, which do not report any increased complication rates during pregnancy or an increased risk of malformations (12-15). The registries for naratriptan (14, 16) and rizatriptan (17) also show similar results. No unfavorable effects of triptans were observed for the child's further motor and emotional development up to the age of 3 (18). The most extensive experience is available for sumatriptan. Sumatriptan (oral, nasal, sc) can therefore be used as the drug of choice for severe migraine attacks during pregnancy. If treatment is ineffective and urgently needed, other triptans can also be used.
Ergotamines are contraindicated in pregnancy. This also applies to Gepante and Lasmiditan.
How can migraines be treated while breastfeeding?
 During breastfeeding, migraines often return to their previous pattern of frequency and severity.
During breastfeeding, migraines often return to their previous pattern of frequency and severity.
Whenever possible, mild migraine attacks during breastfeeding should be treated non-medication through stimulus isolation, rest, relaxation and ice packs. Metoclopramide, dimenhydrinate and ondansetron are not recommended during breastfeeding; the substances are excreted in breast milk and can cause side effects in infants.
The active ingredients and degradation products of acetylsalicylic acid and ibuprofen only pass into breast milk in small quantities. For short-term use, interruption of breastfeeding is usually not necessary. Breast-feeding must be interrupted if used for a longer period of time or if a higher dose is taken. Paracetamol can be administered in therapeutic doses during breastfeeding, but due to its low effectiveness it should only be used when other options are not available.
Breastfeeding infant drug exposure from maternally administered triptans appears to be low and suggests that triptan use is compatible with breastfeeding (19). Sumatriptan can be used while breastfeeding without the need for pumping. Infant exposure is very low at 0.5% of the maternal dose. To date, no adverse effects on breastfed infants have been reported (20). A dose of only 0.002% in breast milk after 24 hours has been described for eletriptan, so this active ingredient can theoretically be considered even safer (21).
There is no clear controlled evidence for the other triptans. As an additional safety measure, it can be recommended to express breast milk before taking it in order to breastfeed the baby. 24 hours after use, breastfeeding should not be carried out and milk should be discarded (22).
literature
- Erdal H, Holst L, Heitmann K, Trovik J. Antiemetic treatment of hyperemesis gravidarum in 1,064 Norwegian women and the influence of European warning on metoclopramide: a retrospective cohort study 2002-2019. BMC Pregnancy Childbirth. 2022;22(1):464.
- Saldanha IJ, Cao W, Bhuma MR, Konnyu KJ, Adam GP, Mehta S, et al. Management of primary headaches during pregnancy, postpartum, and breastfeeding: A systematic review. Headache. 2021;61(1):11-43.
- Zafeiri A, Raja EA, Mitchell RT, Hay DC, Bhattacharya S, Fowler PA. Maternal over-the-counter analgesics use during pregnancy and adverse perinatal outcomes: cohort study of 151 141 singleton pregnancies. BMJ Open. 2022;12(5):e048092.
- Tadokoro-Cuccaro R, Fisher BG, Thankamony A, Ong KK, Hughes IA. Maternal Paracetamol Intake During Pregnancy-Impacts on Offspring Reproductive Development. Front Toxicol. 2022;4:884704.
- Bauer AZ, Swan SH, Kriebel D, Liew Z, Taylor HS, Bornehag CG, et al. Paracetamol use during pregnancy – a call for precautionary action. Nat Rev Endocrinol. 2021;17(12):757-66.
- Patel R, Sushko K, van den Anker J, Samiee-Zafarghandy S. Long-Term Safety of Prenatal and Neonatal Exposure to Paracetamol: A Systematic Review. Int J Environ Res Public Health. 2022;19(4).
- Bar-Oz B, Clementi M, Di Giantonio E, Greenberg R, Beer M, Merlob P, et al. Metamizole (dipyrone, optalgin) in pregnancy, is it safe? A prospective comparative study. Eur J Obstet Gynecol Reprod Biol. 2005;119(2):176-9.
- Schiessl B, Schneider KT, Zimmermann A, Kainer F, Friese K, Oberhoffer R. Prenatal constriction of the fetal ductus arteriosus–related to maternal pain medication? Z Obstetrics Neonatol. 2005;209(2):65-8.
- Weintraub A, Mankuta D. Dipyrone-induced oligohydramnios and ductus arteriosus restriction. Isr Med Assoc J. 2006;8(10):722-3.
- Sanchez de la Nieta MD, Rivera F, De la Torre M, Alcazar R, Caparros G, Paz Alcaide M, et al. Acute renal failure and oligohydramnios induced by magnesium dypirone (metamizole) in a pregnant woman. Nephrol Dial Transplant. 2003;18(8):1679-80.
- Amundsen S, Nordeng H, Nezvalova-Henriksen K, Stovner LJ, Spigset O. Pharmacological treatment of migraine during pregnancy and breastfeeding. Nat Rev Neurol. 2015;11(4):209-19.
- Marchenko A, Etwel F, Olutunfese O, Nickel C, Koren G, Nulman I. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015;55(4):490-501.
- Nezvalova-Henriksen K, Spigset O, Nordeng H. Triptan exposure during pregnancy and the risk of major congenital malformations and adverse pregnancy outcomes: results from the Norwegian Mother and Child Cohort Study. Headache. 2010;50(4):563-75.
- Cunnington M, Ephross S, Churchill P. The safety of sumatriptan and naratriptan in pregnancy: what have we learned? Headache. 2009;49(10):1414-22.
- Loder E. Safety of sumatriptan in pregnancy: a review of the data so far. CNS Drugs. 2003;17(1):1-7.
- Evans EW, Lorber KC. Use of 5-HT1 agonists in pregnancy. Ann Pharmacother. 2008;42(4):543-9.
- Fiore M, Shields KE, Santanello N, Goldberg MR. Exposure to rizatriptan during pregnancy: post-marketing experience up to 30 June 2004. Cephalalgia. 2005;25(9):685-8.
- Wood ME, Frazier JA, Nordeng HM, Lapane KL. Prenatal triptan exposure and parent-reported early childhood neurodevelopmental outcomes: an application of propensity score calibration to adjust for unmeasured confounding by migraine severity. Pharmacoepidemiol Drug Saf. 2016;25(5):493-502.
- Amundsen S, Nordeng H, Fuskevag OM, Nordmo E, Sager G, Spigset O. Transfer of triptans into human breast milk and estimation of infant drug exposure through breastfeeding. Basic Clin Pharmacol Toxicol. 2021;128(6):795-804.
- Soldin OP, Dahlin J, O'Mara DM. Triptans in pregnancy. Ther Drug Monitor. 2008;30(1):5-9.
- David PS, Kling JM, Starling AJ. Migraine in pregnancy and lactation. Curr Neurol Neurosci Rep. 2014;14(4):439.
- Bendtsen L, Birk S, Kasch H, Aegidius K, Sorensen PS, Thomsen LL, et al. Reference program: diagnosis and treatment of headache disorders and facial pain. Danish Headache Society, 2nd Edition, 2012. The journal of headache and pain. 2012;13 Suppl 1:S1-29.
Author: Prof. Dr. Hartmut Göbel







Leave a comment