Migraine is among the most debilitating diseases known to humankind. According to a recent WHO overview, migraine ranks sixth among the most disabling diseases. If medication-overuse headache is included, migraine takes third place. Considering the various forms of the 367 currently known types of headache, migraine and headaches are by far the most severe, frequent, and debilitating diseases in humans.
Current medication-based treatment options for migraine include preventative therapies and the treatment of acute headache attacks. A significant proportion of sufferers do not experience sufficient relief through preventative therapies. Even current attack therapies, with their existing options, may not provide effective relief, and contraindications or intolerances may exist.
Recent studies have provided ample evidence that calcitonin gene-related peptide, or CGRP for short, plays a significant role in the development, maintenance, and chronicity of migraines. CGRP is a neuropeptide consisting of 37 amino acids. It is encoded by the same gene as the hormone calcitonin.
CGRP has therefore become a focus of new migraine treatment options. The development of CGRP receptor antagonists, the so-called gepants, represents a new class of drugs for the acute treatment of migraine attacks. However, their development was halted due to liver toxicity.
Current research is intensively focused on the development of monoclonal antibodies against CGRP. Both vascular and neuronal mechanisms play a role in the complex pathophysiology of migraine. Key neurotransmitters involved in the development of migraine pain include serotonin (5-hydroxytryptamine, 5-HT), nitric oxide, and CGRP. CGRP consists of 37 amino acids and was discovered approximately 30 years ago. It is widely distributed throughout the peripheral and central nervous systems. Every major organ is innervated by nerve fibers containing CGRP. In particular, CGRP and CGRP receptors are found in anatomical structures that are significant for the development of migraine. These include the cerebral cortex, the meninges, the hypothalamus, the cerebellum, and the brainstem. CGRP is also found in many neurons that are important for the trigeminal-vascular pain-processing system. CGRP is found in over 50% of neurons in the trigeminal nerve. It also plays a role in pain processing in the brainstem, leading to increased sensitivity to stimuli. In the periphery, CGRP is released by neurons that innervate blood vessels, particularly in the cardiac and intracranial vessels. CGRP causes pronounced and sustained vasodilation, mediated by the activation of smooth muscle receptors. These processes are crucial in neurogenic inflammation, resulting in vasodilation, sensitization, swelling, and other inflammatory mechanisms.
Initial findings have shown that at the onset of a migraine attack, CGRP leads to dilation of the middle cerebral artery and the middle meningeal artery. Further studies have demonstrated that CGRP triggers and maintains both peripheral and central sensitization. Sensitization is considered a fundamental step in the development of a migraine attack and the chronicity of the disease. The neurogenic inflammation during a migraine attack is modulated by CGRP release directly due to vasodilation and indirectly due to the release of substance P, resulting in plasma extravasation. Additionally, CGRP induces mast cell degranulation and triggers the release of pro-inflammatory and inflammatory substances. The release of cytokinins leads to the sensitization of sensory neurons. As a neuromodulator, CGRP activates synaptic glutamate transmission in the dorsal horn and the trigeminal nucleus. This leads to a further central increase in the sensitization and activation of nociceptive reflexes. Pain behavior is also activated through the activation of central neurons, particularly in the amygdala. Anxiety and avoidance behavior are also affected. The initiation of a migraine attack is associated with cortical spreading depression (CSD). This involves a local reduction or complete interruption of neuronal activity in the cerebral cortex. This depolarization spreads slowly and gradually across the cortex, analogous to the spread of the migraine aura. CSD leads to the release of CGRP, resulting in neurogenic inflammation including sensitization, hyperemia, vasodilation, swelling, and impaired function.
Studies also show that CGRP is involved in the development of hypersensitivity to sensory stimuli, particularly photophobia. Since CGRP can also be extensively released in the enteric nervous system, it is thought that gastrointestinal symptoms such as gastric stasis, nausea, and vomiting are modulated by CGRP mechanisms.
The connection between the development of migraine and CGRP initially emerged from the discovery that stimulation of the trigeminal ganglion leads to the release of CGRP. During a spontaneous migraine attack, elevated concentrations of CGRP are found in the jugular vein. Saliva also shows increased CGRP concentrations during an acute migraine attack. These levels can be reduced by treatment with a triptan. Even between migraine attacks, patients with migraine have elevated CGRP levels.
Intravenous administration of CGRP can directly trigger migraine attacks in migraine patients. Patients who do not suffer from migraines experience headaches without the typical symptoms of a migraine after CGRP administration. It is therefore assumed that migraine patients have a particular sensitivity to CGRP. Finally, selective blockade of the CGRP receptor can effectively terminate an acute migraine attack.
CGRP receptor antagonists
Based on these findings, various approaches have been systematically pursued in recent years to therapeutically utilize the new insights into CGRP in migraine pathophysiology. Initially, the so-called gepants, CGRP receptor antagonists, were developed as a completely new class of migraine medication. Their mechanism of action was based on the competitive inhibition of endogenous CGRP at the CGRP receptor. Six different gepants have now been developed and tested in clinical trials. The results showed a clinical efficacy that significantly exceeded that of placebos, but was comparable to that of triptans. A key advantage of gepants is that, unlike triptans, they do not cause vasoconstriction. The development of CGRP antagonists was discontinued due to liver toxicity during long-term use and because their efficacy was not superior to that of triptans. However, their mechanism of action demonstrated the important role of CGRP in migraine pathophysiology and the possibility of using CGRP in the treatment of migraine.
Monoclonal antibodies against CGRP
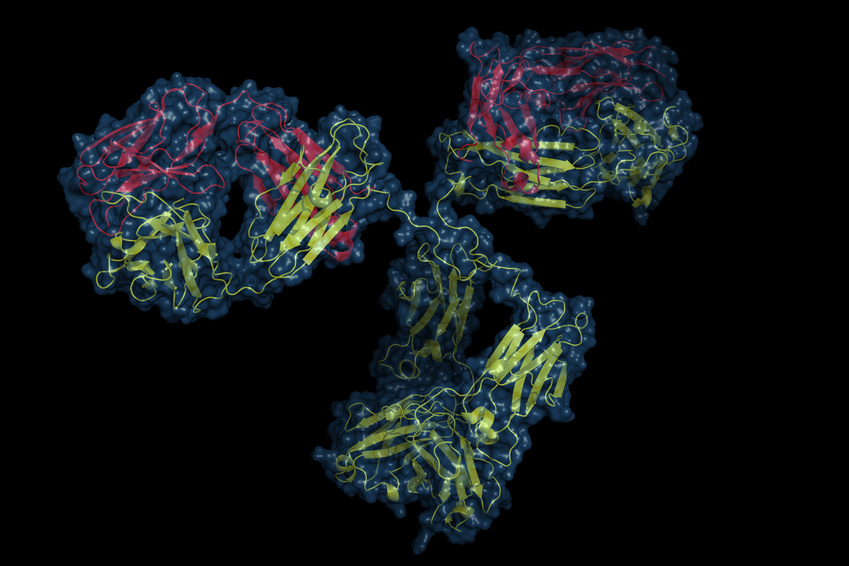
The biological effects of CGRP can alternatively be blocked by monoclonal antibodies against CGRP itself or against the CGRP receptor. Currently, four monoclonal antibodies for the preventive treatment of episodic or chronic migraine are in clinical development programs. Three monoclonal antibodies target the neuropeptide itself: ALD-403 (Alder Biopharmaceuticals), LY2951742 (developed by Arteau's Therapeutics and further developed by Eli Lilly), and LBR-101, currently known as TEV-48125 (developed by Labrys Biologics-Pfizer, acquired by Teva Pharmaceuticals). A fourth monoclonal antibody targets the CGRP receptor itself: AMG334 (Amgen, Inc.; further developed by Novartis).
ALD403 is administered as an infusion every three months. Results from a Phase 2b trial for the prevention of chronic migraine are expected in July 2016. A Phase 3 trial is investigating the prevention of frequent episodic migraine. This trial is scheduled to conclude in April 2017. Another Phase 3 trial is planned to begin in 2016. Additionally, a Phase 2b trial will analyze self-administration of ALD403 by patients with episodic migraine.
TEV-48125 is currently being investigated in a Phase 3 trial. The analysis of its efficacy in chronic migraine is expected to be completed in October 2017.
LY2951742 was investigated in a Phase 2 study completed in August 2015. The study analyzed the preventive effect of subcutaneous administration of the drug every four weeks for a period of 12 weeks on episodic migraine. Significant efficacy was demonstrated compared to placebo in reducing the number of migraine days. Further Phase 3 studies are currently underway. A study investigating efficacy in episodic migraine is expected to be completed by December 2017. Another study investigating efficacy in chronic migraine is expected to be completed in April 2018. An additional open-label, long-term study is expected to be completed in September 2017.
AMG334 is currently being investigated in a Phase 2 trial regarding its preventive efficacy in chronic migraine. Long-term efficacy and safety will be analyzed in a further Phase 2 trial, which is expected to be completed by July 2017. Two Phase 3 trials investigating its preventive efficacy in episodic migraine are scheduled for completion by October 2017 and February 2018, respectively.
Compatibility and safety
Given the experience gained with the development of CGRP antagonists, the results regarding the tolerability and safety of monoclonal antibodies against CGRP must be awaited in long-term studies. These antibodies do not selectively block CGRP-mediated vasodilation throughout the body. Their effects on the inhibition of cardiovascular vasodilation, e.g., during stress or ischemia, as well as their interactions in the treatment of arterial hypertension, remain unclear. Interactions with cardiac and cerebral blood flow are conceivable. However, previous studies have shown no effects on the ECG or other hemodynamic parameters. The potential induction of immunological reactions in patients upon administration of these monoclonal antibodies also remains an open question.
effectiveness
Undoubtedly, current preventive treatment options for migraine are unsatisfactory for many patients. Monoclonal antibodies have also proven ineffective in some patients studied in previous clinical trials. For example, 47% of patients with a low dose and 45% with a high dose of TEV-41825 did not achieve a 50% reduction in migraine frequency in terms of headache days. It remains true that migraine is a complex disease mediated by a wide variety of pathophysiological pathways and diverse molecules. The role of CGRP varies from patient to patient. However, there appears to be a subgroup of patients who respond very effectively to treatment with monoclonal antibodies against CGRP. More than 15% of treated patients reported a complete cessation of migraine attacks. Therefore, this treatment will offer highly effective options for some patients, while others will not benefit sufficiently. Furthermore, the comparative efficacy with existing preventive medications remains to be seen. However, one thing is already clear: migraine prevention must encompass a variety of strategies. A differentiated, individualized therapy is necessary in every case, especially for severe and chronic cases. Even with monoclonal antibodies, a treatment that eliminates migraines with a single injection and allows one to live as one wishes is not to be expected.










I've suffered from migraines for 30 years. At times, I had two attacks, three days a week. With only seven days in a week, that doesn't leave much usable time.
I also agree with the comments about volunteering for a study immediately.
Because of the numerous medications I take, I've already had to undergo several months of withdrawal due to medication-induced headaches.
I'm hoping for an improvement so I don't lose my job.
I agree with Ms. Gilles. I'm almost 50 and have been struggling with migraines since my teens. My hope that menopause would bring improvement hasn't materialized. If you need study participants, I'm in!
I too have suffered from severe migraines and tension headaches for 28 years. Currently, I am experiencing another period of intense pain, to the point that I am unable to work. I am 57 years old and have almost given up all hope of being pain-free. I would be very happy if I could test this antibody injection.
I've suffered from migraines for 45 years and have become unable to work due to chronic migraines. Currently, I have them 10-15 days a month. Thanks to Ascotop, I can manage them to some extent now.
I would immediately volunteer to participate in studies.
I continue to hope for a vaccine and am worried about how things will be with medication as I get older (I'm almost 60).
It would be wonderful if I could live to see this revolution.