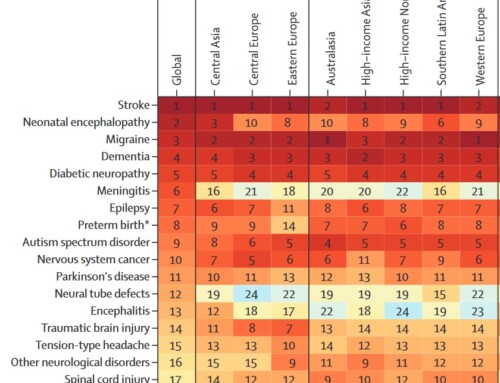
The Kiel Pain Clinic receives hundreds of inquiries daily regarding a possible link between a Covid-19 vaccination and the management of concurrent migraine treatment. Here are the most important answers:
What is known about interactions between Covid-19 vaccinations and simultaneous migraine therapy?
Currently, there are no known results from studies indicating any interactions or complications between COVID-19 vaccination and an additional existing preventive migraine treatment or acute treatment of a migraine attack.
Does this also apply to onabotulinumtoxin A (Botox) injections and CGRP monoclonal antibodies?
This applies to both onabotulinumtoxin A (Botox) injections and the use of CGRP monoclonal antibodies.
Can migraine treatment weaken the effectiveness of the vaccine?
There is currently no data to suggest that migraine treatment impairs the efficacy or safety of COVID-19 vaccines. Likewise, there is no evidence that vaccination against COVID-19 reduces the efficacy of migraine treatment.
There is currently no data showing that the antibodies against the SARS-CoV-2 spike protein produced by the vaccine would render the drug onabotulinumtoxin A ineffective. This also applies to the drugs erenumab (Aimovig), fremanezumab (Ajovy), or galcanezumab (Emgality).
Is it safe to undergo vaccination and migraine treatment simultaneously?
Just because something is unknown or hasn't been studied doesn't mean that risks don't exist and that one can proceed without hesitation. This is especially true regarding the potential for side effects to be masked when used concurrently. Concerns arise less from the effectiveness of the medication itself than from the possible interaction of side effects.
What side effects can Covid-19 vaccines have?
Using the BNT162b2 mRNA Covid-19 vaccine (BioNTech/Pfizer) as an example, the most common side effects are injection site reactions (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%) and fever (14.2%).
Using the AstraZeneca ChAdOx1 nCoV-19 (AZD1222) vaccine as an example, the most common adverse reactions are injection site pain (54.2%), headache (52.6%), fatigue (53.1%), muscle pain (44.0%), joint pain (26.4%), malaise (44.2%), nausea (21.9%), chills (31.9%), or fever > 38°C (7.9%). In the clinical trials for the AstraZeneca vaccine, the use of paracetamol before vaccination was recommended as a preventative measure in all studies (except study COV005; this was introduced as a change during study COV001). Those vaccinated were advised to take 1000 mg of paracetamol after vaccination and to continue this preventative measure every six hours for 24 hours to reduce vaccine-induced adverse reactions. It can therefore be assumed that the prophylactic administration of paracetamol alleviated side effects such as fever and headaches. Preventive treatment of headaches after vaccination without symptoms is not recommended.
What side effects can monoclonal antibodies have for migraine prevention?
When using monoclonal antibodies for migraine prevention, the following side effects can occur, using erenumab (Aimovig) as an example: hypersensitivity reactions, such as acute severe allergic reactions affecting multiple or all areas of the body (anaphylaxis), spontaneous swelling of the skin and mucous membranes (angioedema), rash, swelling, fluid retention (edema), hives (urticaria), constipation, itching (pruritus), immune-mediated rash with itching, muscle cramps, and injection site reactions.
Is it possible to reduce the overlap of side effects?
Since pronounced side effects can occur in individual cases, we recommend maintaining as long an interval as possible between the Covid-19 vaccination and the administration of monoclonal antibodies for migraine prevention to avoid additive effects (summarization of side effects from both medications). As the administration of monoclonal antibodies for migraine prevention is usually given four weeks apart, this corresponds to a possible interval of 14 days. This recommendation is based on the need to avoid overlapping side effects from both applications.
What can you do about headaches after vaccination?
If headaches occur after vaccination, they can be treated with aspirin, ibuprofen, or paracetamol. Preventive treatment of headaches after vaccination without symptoms is not recommended.
How can migraine attacks be treated after vaccination?
If migraine attacks occur after vaccination, they can be treated as usual with the recommended acute medication (e.g. triptans, painkillers).






A very helpful comment, not just for migraine sufferers. Thank you for that. I will also inform my circle of friends about it.
Thanks for the tip. I'll follow the recommendation for the second vaccination. I already had the first AstraZeneca vaccination and, purely by chance, I kept a 12-day interval between it and the CGRP injection. With Aimovig, as usual, I had almost no side effects, but with the AstraZeneca vaccination, I experienced pronounced fatigue and a feeling of weakness that lasted a very long time; otherwise, nothing, not even a headache.