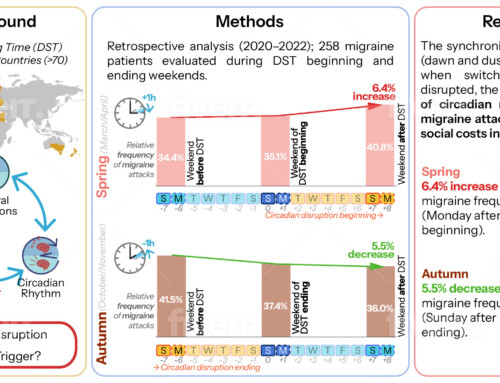
Press release from STIKO from March 30, 2021: “The task of STIKO is to continuously evaluate all scientific data on the effectiveness and safety of vaccines and to derive recommendations from it.
Of course, this also applies if new information is gained about the safety of a vaccine that was not previously available because the number of vaccinated people in the approval studies was not sufficient to detect this. After several consultations, the STIKO, with the involvement of external experts, decided by a majority to only recommend the COVID-19 Vaccine AstraZeneca for people aged 60 and over based on the currently available data on the occurrence of rare but very serious thromboembolic side effects This side effect occurred 4 to 16 days after vaccination, predominantly in people aged <60 years.
With regard to the question of administering the second vaccine dose for younger people who have already received a first dose of the COVID-19 Vaccine AstraZeneca, the STIKO will make a supplementary recommendation by the end of April. Since vaccination with the AstraZeneca vaccine began at the beginning of February, the first second vaccinations are scheduled for the beginning of May, with a recommended vaccination interval of 12 weeks.
The agreed draft resolution and the scientific justification are currently in the comment process with the federal states and the affected specialist groups. The resolution will be passed on Thursday, April 1, 2021, after reviewing the responses and renewed consultation with STIKO. In principle, there is a possibility that there will be changes to the draft recommendation after the commenting process.”
The European Medicines Agency (EMA) examined the matter and recommended continued vaccination with the AstraZeneca vaccine on March 18, 2021. On March 18, 2021, the federal and state health ministers decided to resume vaccinations with the AstraZeneca vaccine from March 19, 2021. The justification for the approach was that the benefits of the vaccine in combating the still widespread threat of COVID-19 continue to outweigh the risk of side effects. A warning has been included in the product information.
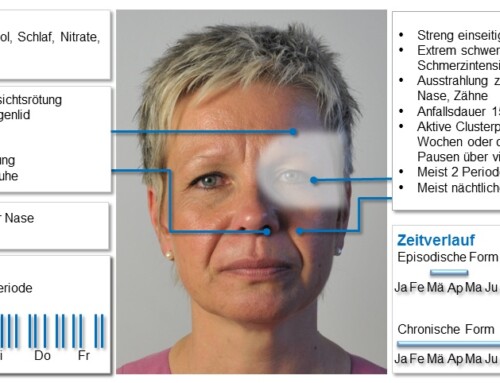
A Rote Hand letter dated March 24, 2021 explains: “Those who have been vaccinated should be instructed to see a doctor immediately if they develop symptoms such as shortness of breath, chest pain, leg swelling or persistent abdominal pain after vaccination. “In addition, anyone who experiences neurological symptoms after vaccination, such as severe or persistent headaches or blurred vision, or who develops skin bruising (petechiae) outside the site of vaccination after a few days, should seek medical attention immediately.”
Download: Red Hand Letter COVID-19 Vaccine AstraZeneca: Risk of thrombocytopenia and coagulation disorders
In this context, the question arises as to why vaccination with the AstraZeneca vaccine in Germany was restricted to people under 65 years of age, contrary to approval by the EMA (European Medicines Agency), citing insufficient efficacy data in older people. Young women in particular in the health system , nurses, doctors, etc., have been given priority treatment with this vaccine. It remains to be seen what benefit an additional warning in the package insert has regarding complications if no alternatives are available. This would be understandable if there had been no other options. However, these were available with Biontech/Pfizer and other active ingredients. According to current findings, young women belong to the special risk group of the complications described. On March 29, 2021 the Euskirchen district decided with immediate effect not to vaccinate women younger than 55 years with the AstraZeneca active ingredient. According to its announcement dated March 26, 2021, following the death of a 49-year-old nurse, the Rostock University Clinic is also suspending AstraZeneca vaccinations for people with high blood pressure, overweight and women who take the pill. The Canadian expert committee for the corona vaccination campaign is also currently recommending the suspension of Astrazeneca's drug for people under the age of 55. According to a report from March 30, 2021, the state-owned Charité and Vivantes clinics in Berlin are also suspending vaccinations with the Astrazeneca preparation for women under 55 years of age. After Berlin, the city of Munich also announced on August 30, 2021 that it would no longer vaccinate people under 60 with AstraZeneca until further notice. On March 30, 2021, the directors of five university clinics in North Rhine-Westphalia also called for a temporary stop to vaccinations of younger women with the AstraZeneca vaccine. And also today on March 30, 2021, the Standing Vaccination Commission (Stiko) announced: “Based on the currently available, although still limited, evidence and taking into account the current pandemic situation, Stiko recommends the Covid-19 Vaccine AstraZeneca for people of old age Use for 60 years. However, their use below this age limit remains possible at the doctor's discretion and with individual risk acceptance after careful information. Stiko will comment on the second vaccine dose for younger people who have already received a first dose of the Covid-19 Vaccine AstraZeneca by the end of April.”








Leave a comment