July 30, 2018. Migraine immunotherapy with the active ingredient erenumab (Aimovig) can now also be marketed in the EU. It was initially approved in the US in May 2018, followed by approval in Switzerland in July. Now, on July 30, 2018, the European Commission has also granted market access to erenumab in the EU. On May 31, 2018, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) issued a recommendation for the marketing authorization of erenumab (Aimovig) for the prophylaxis of migraine attacks. The drug was already approved by the US Food and Drug Administration (FDA) on May 17, 2018, for the preventive treatment of migraine in adults in the US (see this article ). Aimovig is available as a 70 mg solution for subcutaneous injection. The active ingredient in Aimovig is erenumab. The drug works by binding to the calcitonin gene-related peptide (CGRP) receptor. Studies have shown that Aimovig can reduce the number of monthly migraine days. The most common side effects were injection site reactions and constipation.
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has now published the indication text. According to this text, Aimovig is indicated for the prevention of migraine in adults who experience at least four migraine days per month. The drug can therefore be used for the prevention of both episodic and chronic migraine.
The European Medicines Agency (EMA) has recommended that Aimovig can be prescribed by doctors specializing in the diagnosis and treatment of migraine. Further details regarding prescribing will be published in all European languages once the European Commission has granted marketing authorization.
The next step was for the European Commission to evaluate the recommendation of the European Medicines Agency (EMA). This has now been completed. The recommendation applies to all 28 member states of the European Union, including Iceland, Norway, and Liechtenstein. The drug is therefore expected to be available in Germany in August/September 2018. The manufacturer has established a patient access program in the USA. This program allows patients to receive the drug free of charge for two months, or to have their co-payment limited to $5 per month.
Summary of the EMA recommendation on erenumab
US product information
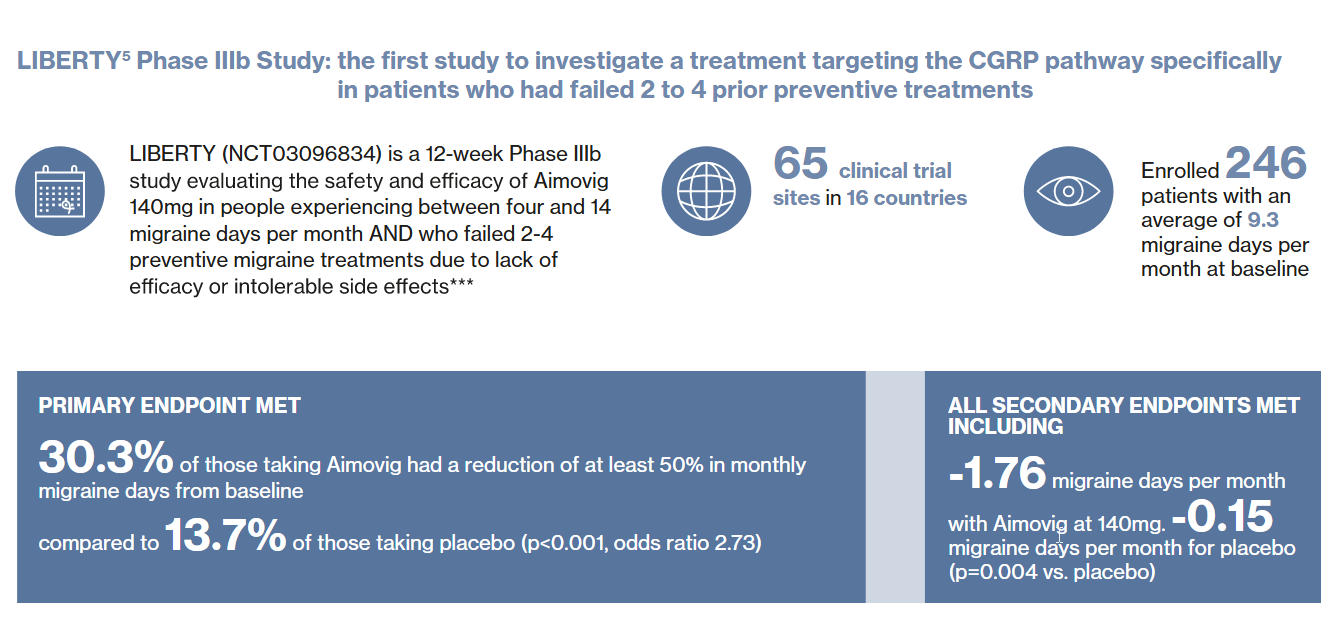
The figures show summaries of the results from the clinical trial program for erenumab, on which the approval is based. A total of over 3,000 migraine patients participated in the clinical trials. The long-term program currently includes over 5 years of data on safety and tolerability. During this period, the safety profile was comparable to that of placebo treatment.









I hope that cluster patients can also benefit from this, that would be a dream :)
Finally
I really hope it helps. I currently have 15 migraine days a month, strength 8 to 10, and have really tried everything in my migraine career.
That gives hope.
A very important step for us migraine sufferers!!
I'm very excited about the future.
Olaf Biewald