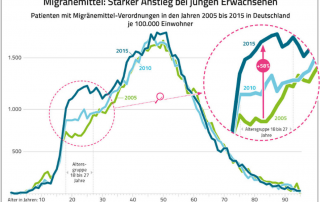
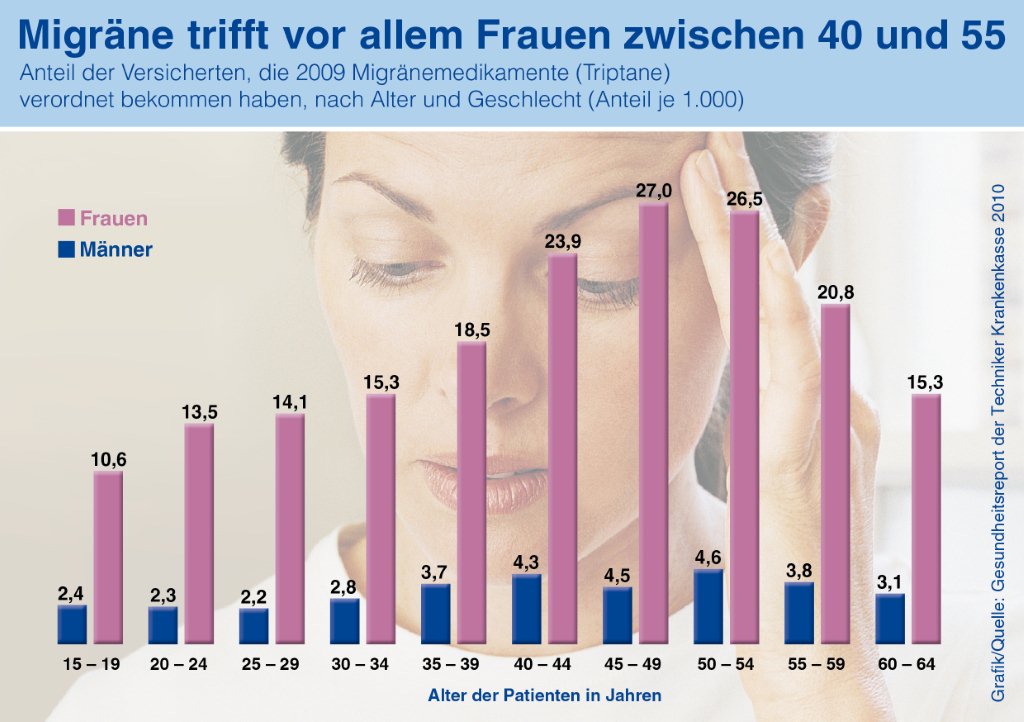
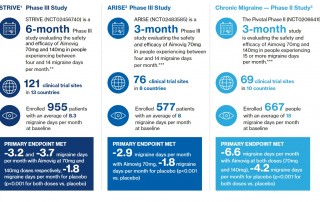
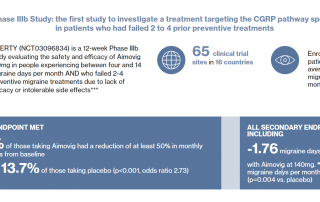
Current press information on migraine and headache research, migraine and headache diagnostics, migraine and headache therapy
Goodbye headache - The book for children and parents against migraines and headaches
Prof. Dr. Hartmut Göbel Migraines and headaches are a widespread disease. They either put a strain on you. Or you at least know a lot of people around you who suffer from headaches. Even small children can often [...]